When it comes to manufacturing medical devices, compliance to FDA regulations is of paramount importance. The FDA 21 CFR Part 820 (also known as the Quality System Regulation) outlines the Current Good Manufacturing Practices (CGMP) for the design and production of medical devices. The FDA 21 CFR Part 820, referred to as the Quality System Regulation, defines current good manufacturing practices (CGMP) requirements in the development, production and distribution of medical devices.
Understanding FDA 21 CFR Part 820 Compliance
FDA 21 CFR Part 820 is a set of complete requirements for manufacturers of medical devices to follow for safety and quality. These requirements encompass various aspects of the manufacturing process such as design control, document control preventive and corrective actions (CAPA) as well as production and process controls as well as other aspects. In observing these regulations, manufacturers demonstrate their commitment to manufacturing high-quality, safe and effective medical devices.
Manufacturers of medical devices be faced with a lot of challenges in navigating the FDA 21 CFR 820 regulations. It can be difficult to keep track of all the requirements and the documentation. Due to the nature of the industry being fluid, it is essential to be able to adapt rapidly to regulatory updates or changes. Manufacturers need strong tools and systems to ensure they adhere to FDA regulations and to streamline their compliance processes.
What are the functions of QT9 Software?
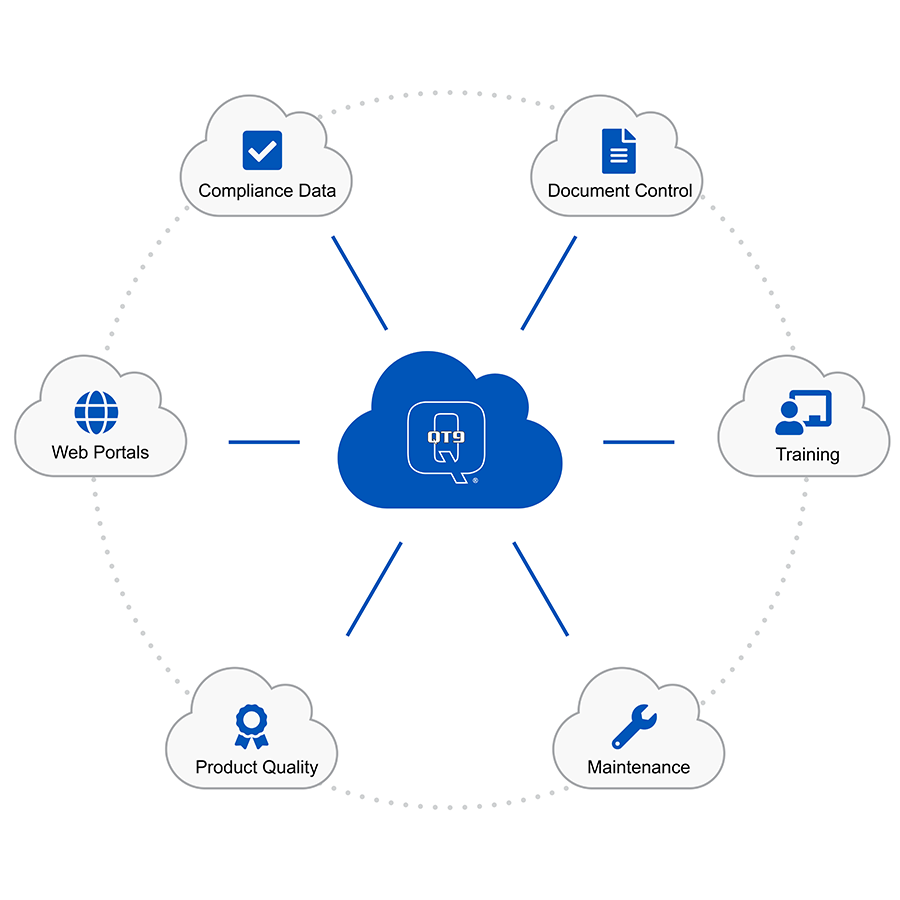
QT9 QMS software can provide a complete solution for medical device manufacturers looking to maintain and achieve FDA 21 CFR Part 820 conformity. This quality system was specially developed to meet the demands of the medical device industry. It is a suite of features and tools that enhances overall quality management and helps to ensure compliance.
QT9 QMS’s ability to streamline compliance is one of the software’s key advantages. The software centralizes quality-related documentation and processes, enabling manufacturers control and monitor compliance. QT9 is a single platform for managing all aspects of FDA 21 CFR Part 820 compliance, including design control and risk management, supplier control and audit management.
QT9 QMS provides manufacturers with real-time reports, which enable them to gain valuable insights about their compliance status and performance. Dashboards provide a comprehensive overview of the metrics used to measure compliance, nonconformances and remedial measures. It allows the stakeholders to make informed decisions and take proactive actions to fix any problems. This transparency is crucial in maintaining continuous compliance and driving on-going improvement efforts.
The ability to customize and adapt
QT9 QMS provides a superior degree of customization that allows manufacturers to adapt the system to meet their unique compliance needs and organizational process. When it comes to defining workflows, creating forms that are custom-designed, or setting roles and access rights for users the software can be tailored to the specific demands of manufacturers. This flexibility will ensure that compliance efforts are aligned to the particulars of the organization, ultimately enhancing efficiency and effectiveness.
Medical device manufacturers can boost their compliance efforts by leveraging QT9 QMS. The software streamlines quality management processes to lessen the amount of manual work and allow teams to focus more on strategic projects. The centralized nature QT9 QMS encourages collaboration and communication between departments. This allows for a cohesive approach to compliance and quality management.
Incorporating the Future of Compliance
It is vital to be aware of the ever-changing regulatory landscape and adapt to new changes. The QT9 software provides medical device manufacturers the means to be ready for the future and stay compliant. They are also able to adapt to changes in the regulatory environment. It provides manufacturers with options like automated compliance updates with customizable alerts as well as the ability to respond swiftly and efficiently in the face of regulatory changes.
FDA 21 CFR part 820 compliance, therefore, is an important element of the medical device industry. Making and maintaining FDA compliance is essential to ensure the safety and efficacy of medical devices. QT9 QMS is a dependable tool for compliance that provides an adaptable and powerful solution to help streamline compliance processes. It also offers real-time transparency that improves the efficiency and effectiveness of compliance. As the landscape of manufacturing medical devices continues to evolve QT9 QMS is at the forefront, enabling manufacturers to navigate the complexities of compliance with ease and speed.
